Our therapeutic targets are a result of years of real-world evidence.
We're leading a revolution
with PermaFusion® Technology

*Liquid nanodroplets containing biologically active ingredients suspended in petrolatum without an emulsifier.
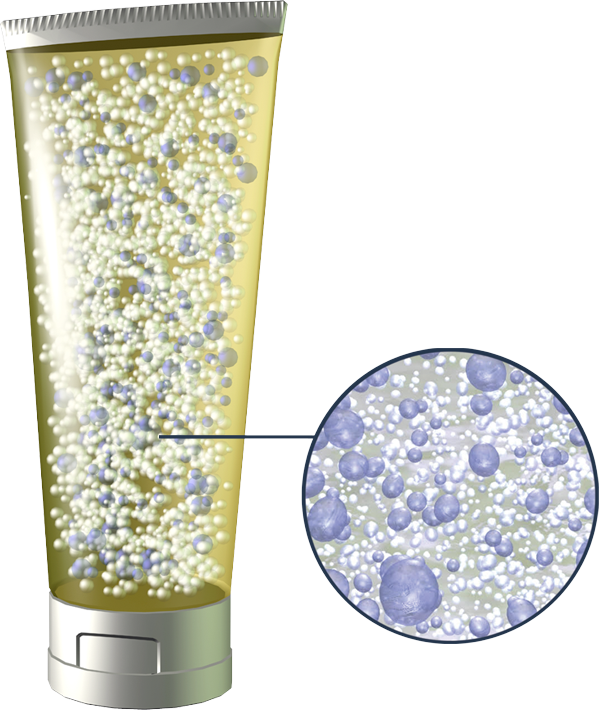
Permafusion is a multi-patented process by which liquid active ingredients are permanently suspended as nanodroplets within petrolatum carriers without additional emulsifiers or binding agents. Permafusion is also ingredient-agnostic, meaning any liquid or liquid soluble active ingredient can be employed with Turn’s delivery technology.
*Liquid nanodroplets containing biologically active ingredients suspended in petrolatum without an emulsifier.
Watch PermaFusion® in action
PermaFusion® is a revolution in topical delivery that maximizes effectiveness while minimizing risk. PermaFusion® is a first of its kind, liquid-in-oil suspension technology that quite literally fuses water and oil. Our revolutionary technology permits use of less active ingredient without compromising effectiveness. Less active ingredient means less toxicity, irritation, and/or sensitization, as has been shown in clinical testing.





